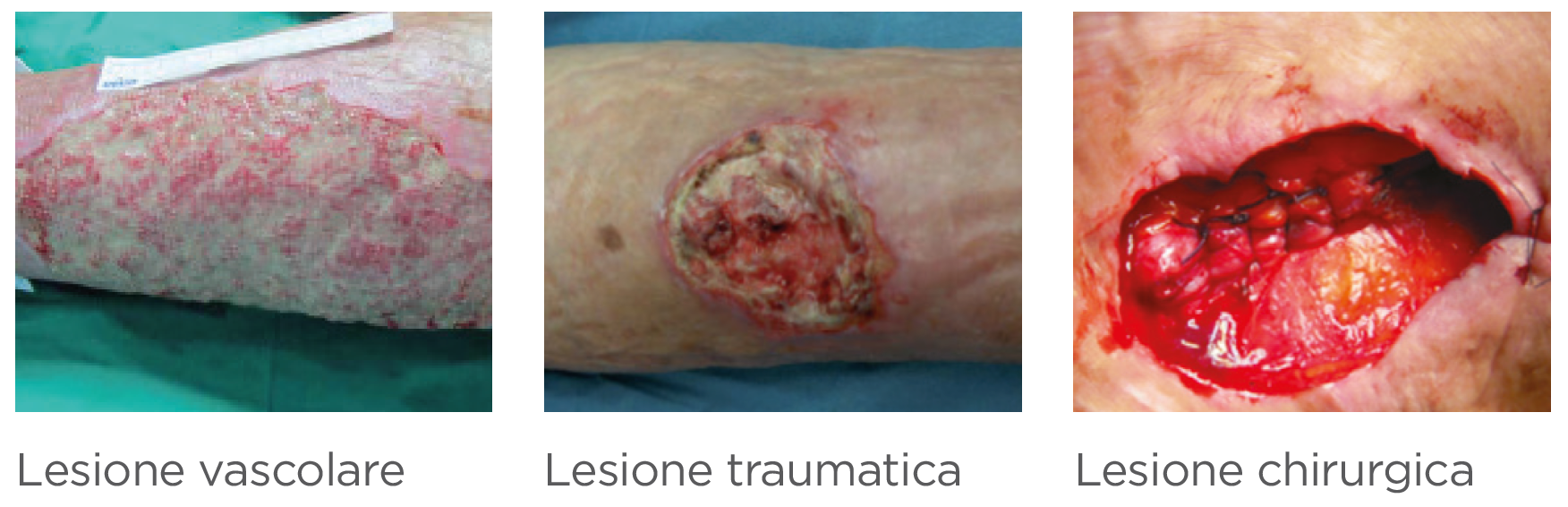
Leg ulcers, diabetic foot ulcers and pressure ulcers take an average of 210 days to heal.(1)
UrgoStart Plus Pad is a local treatment that is effective at every wound phase to reduce healing time by an average of 100 days.(4)
PROPERTIES
UrgoStart Plus Pad is effective at every wound healing phase, from the start* and through to complete healing.
This dressing:
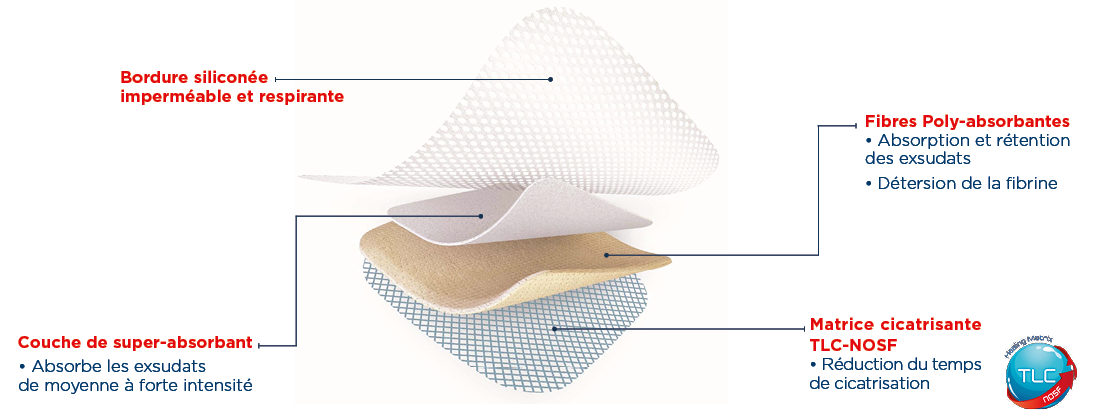
- Desloughs and absorbs exudates thanks to its polyabsorbent fibres.
- Reduces healing time thanks to its patented TLC-NOSF** matrix.
The efficacy of TLC-NOSF on the reduction of healing time has been demonstrated in double-blind, randomised controlled clinical studies (2,3) and by an analysis of observational studies.(4) The earlier TLC-NOSF treatment is started, the more effective it is(3,4).
UrgoStart Plus Pad is highly conformable and can be cut to fit the shape and size of the wound. It is suitable for damaged surrounding skin. It can also be juxtaposed and used under compression.
Finally, UrgoStart Plus Pad is always used as part of a global management approach, along with appropriate etiological treatment.
INDICATIONS
UrgoStart Plus Pad is a dressing indicated for all healing phases* (from the desloughing phase* through to hea
ling) in the treatment of chronic exuding wounds (leg ulcers, pressure ulcers, diabetic foot ulcers) and acute wounds that have become chronic.

Contraindications:
- UrgoStart Plus Pad helps control minor bleeding. However, it should not be used for heavily bleeding wounds.
- To prevent any risk of delay in appropriate treatment, UrgoStart Plus Pad is contraindicated in cancerous wounds and in wounds demonstrating deep abscess formation.
- Do not use UrgoStart Plus Pad in the event of known sensitivity to the dressing.
INSTRUCTION FOR USE
. Wound preparation:
- Clean the wound using the conventional care protocol, then rinse with normal saline.
- If an antiseptic has previously been used, rinse the wound carefully with normal saline before applying UrgoStart Plus Pad.
- Carefully dry the skin around the wound.
- The use of UrgoStart Plus Pad does not dispense with the need for mechanical debridement when required.
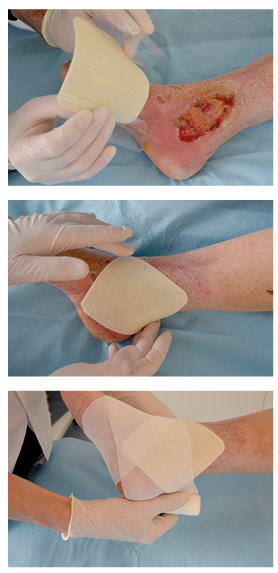
- Dressing application:
- Carefully remove the protective tabs.
- Apply with the micro-adherent side of the UrgoStart Plus Pad dressing in contact with the wound. UrgoStart Plus Pad can be cut using sterile scissors to fit the dressing size to the wound if required.
- If necessary, cover with a secondary dressing suitable for the location and exuding nature of the wound.
- Apply a compression bandage over the dressing where prescribed.
- Dressing removal:
- Pressing on healthy skin, lift a corner of the dressing and remove it carefully.
- Dressing changes:
- Remove the entire dressing when it is saturated and clean the wound if required. It is recommended that the UrgoStart Plus Pad dressing be changed every 1 to 2 days during the wound desloughing phase, then as often as required by the volume of exudates and the clinical progress of the wound. It can be left in place for up to 7 days.
- Discard any unused parts of the dressing.
Precautions for use:
- The micro-adherent mass of the UrgoStart Plus Pad dressing sticks to latex surgical gloves. Therefore it is recommended that the tabs be used to facilitate application of the dressing.
- If the wound shows signs of local infection, it is recommended that the bacterial component be treated first with an antimicrobial dressing (such as UrgoClean Ag) before starting treatment with UrgoStart Plus Pad.
- In the event of an atypical ulcer demonstrating induration or excessive granulation, treatment with UrgoStart Plus Pad should only be started after having verified the absence of any ulcer deterioration, to prevent any delay in diagnosis.
- In the absence of clinical data in the context of Epidermolysis bullosa (irrespective of its duration), the use of UrgoStart Plus Pad dressings is not recommended.
- Stinging or even painful sensations can be reported at the start of treatment: these are generally related to the healing process and rarely warrant suspension of treatment.
- During desloughing, the wound may appear to get larger due to gradual elimination of slough.
- The concomitant use of a cream, lotion, ointment or emulsion is not recommended.
- Sterile individual packaging, for single use: reuse of a disposable dressing can lead to risks of infection.
- Do not re-sterilise the dressing.
- Check that the sterility protector is intact before use. Do not use if the packaging is damaged.
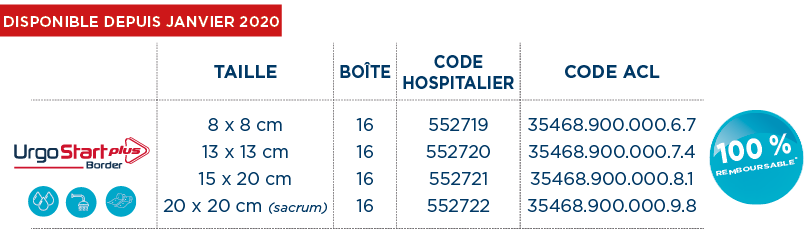
FORMATS
CLINICAL EVIDENCES

In 2012, the Journal of Wound Care published the CHALLENGE(5) study, demonstrating that TLC-NOSF treatment leads to a greater reduction in wound surface area compared to a neutral dressing.
In December 2018, The Lancet Diabetes & Endocrinology international medical journal published the EXPLORER(2) clinical trial, demonstrating that TLC-NOSF treatment heals 60% more patients compared to a neutral dressing and that the earlier TLC-NOSF treatment is started, the more effective it is(3).

The REALITY(4) analysis, published in 2017 in the Journal of Wound Care, presents a compilation of eight observational studies conducted in more than 10,000 patients and shows that TLC-NOSF reduces the healing time of chronic wounds by an average of 100 days and that the earlier TLC-NOSF treatment is started, the more effective it is.
In 2020, the Journal of Wound Care published the last URGOSTART PLUS OBSERVATIONAL STUDY conducted in 1,140 patients, demonstrating that UrgoStart Plus is effective irrespective of the wound type, whatever the wound phase and however long it has been present(6).
 The HAS (French National Authority for Health) has granted the entire range a level III clinical added value (CAV)(7) something that is unprecedented for a dressing.
The HAS (French National Authority for Health) has granted the entire range a level III clinical added value (CAV)(7) something that is unprecedented for a dressing.

The URGO Group was awarded the 2018 Galien France medical device prize for UrgoStart®. Each year, this prestigious award recognises exceptional innovations in the field of health.
 In January 2019, the United Kingdom’s NICE (National Institute for Health and Care Excellence) recommended TLC-NOSF treatment for the wound care of diabetic foot ulcers and venous leg ulcers(8). The NICE recommendations support the fact that the UrgoStart® range has better results in terms of reducing wound healing time, improving patients’ quality of life and enabling significant savings for health authorities compared to neutral dressings.
In January 2019, the United Kingdom’s NICE (National Institute for Health and Care Excellence) recommended TLC-NOSF treatment for the wound care of diabetic foot ulcers and venous leg ulcers(8). The NICE recommendations support the fact that the UrgoStart® range has better results in terms of reducing wound healing time, improving patients’ quality of life and enabling significant savings for health authorities compared to neutral dressings.
 2019: IWGDF (International Working Group on the Diabetic Foot) guidelines This is the very first time that a dressing has been recommended by the IWGDF(9).
2019: IWGDF (International Working Group on the Diabetic Foot) guidelines This is the very first time that a dressing has been recommended by the IWGDF(9).








 UrgoClean, for complete and continuous cleansing
UrgoClean, for complete and continuous cleansing