Leg ulcers, diabetic foot ulcers and pressure ulcers take an average of 210 days to heal.(1)
UrgoStart Plus Border is a local treatment that is effective at every wound phase to reduce healing time by an average of 100 days.(4)
PROPERTIES
UrgoStart Plus Border is effective at every wound healing phase*, from the start* and through to complete healing.
* excluding dry necrosis
This dressing:
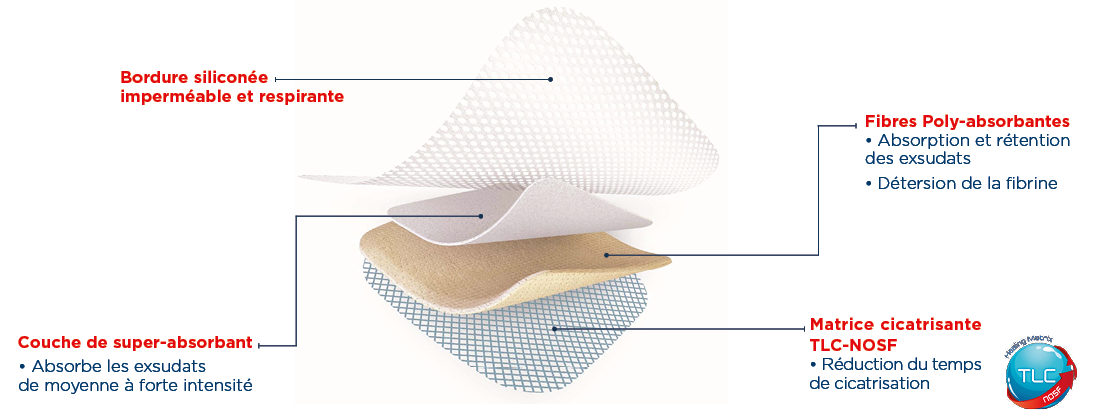
– Desloughs and absorbs exudates thanks to its polyabsorbent fibres.
– Reduces healing time thanks to its healing TLC-NOSF** matrix.
The efficacy of TLC-NOSF on the reduction of healing time has been demonstrated in double-blind, randomised controlled clinical studies (2,3) and by an analysis of observational studies.(4) The earlier TLC-NOSF treatment is started, the more effective it is(3,4).


UrgoStart Plus Border is highly conformable and easy to reposition. Ready to use, it is suitable for both healthy and fragile surrounding skin.
Finally, UrgoStart Plus Border is always used as part of a global management approach, along with appropriate etiological treatment.
INDICATIONS
UrgoStart Plus Border is a dressing indicated for all healing phases* (from the desloughing phase* through to healing) in the treatment of chronic exuding wounds (leg ulcers, pressure ulcers, diabetic foot ulcers) and acute wounds that have become chronic.
*excluding dry necrosis
Contraindications:
- UrgoStart Plus Border helps control minor bleeding. However, it should not be used for heavily bleeding wounds.
- To prevent any risk of delay in appropriate treatment, UrgoStart Plus Border is contraindicated in cancerous wounds and in wounds demonstrating deep abscess formation.
- Do not use UrgoStart Plus Border in the event of known sensitivity to the dressing.
INSTRUCTIONS FOR USE
1. Wound preparation:
- Clean the wound using the conventional care protocol, then rinse with normal saline.
- If an antiseptic has previously been used, rinse the wound carefully with normal saline before applying UrgoStart Plus Border.
- Carefully dry the skin around the wound.
- The use of UrgoStart Plus Border does not dispense with the need for mechanical debridement when required.
2. Dressing application:
- Carefully remove the protective tabs.
- Apply the micro-adherent central pad of UrgoStart Plus Border over the wound (the adhesive silicone edges must be at least 1 cm from the wound). Smooth the dressing.
- Apply a compression bandage over the dressing where prescribed.
Application of the Sacrum format:
- Position the dressing with the tip pointing downwards towards the bottom of the sacral region.
3. Dressing removal:
- Pressing on healthy skin, lift a corner of the dressing and remove it carefully.
4. Dressing changes:
- Remove the entire dressing when it is saturated and clean the wound if required. It is recommended that the UrgoStart Plus Border dressing be changed every 1 to 2 days during the wound desloughing phase, then as often as required by the volume of exudates and the clinical progress of the wound. It can be left in place for up to 7 days.
- Discard any unused parts of the dressing.
Precautions for use:
- The central pad, which includes a super-absorbent layer, should not be cut.
- However, the adhesive silicone edges can be cut using sterile scissors to fit to different anatomical contours.
- If the wound shows signs of local infection, it is recommended that the bacterial component be treated first with an antimicrobial dressing (such as UrgoClean Ag) before starting treatment with UrgoStart Plus Border.
- In the event of an atypical ulcer demonstrating induration or excessive granulation, it is recommended that treatment with UrgoStart Plus Border only be started after having verified the absence of any ulcer deterioration, to prevent any delay in diagnosis.
- In the context of Epidermolysis bullosa (irrespective of its duration), the use of UrgoStart Plus Border dressings is not recommended.
- Stinging or even painful sensations can be reported at the start of treatment: these are generally related to the healing process and rarely warrant suspension of treatment.
- During desloughing, the wound may appear to get larger due to gradual elimination of slough.
- Shave or cut excess hair as close as possible to the skin to ensure good contact with the wound.
- In the event of concomitant use with a cream, lotion, ointment or emulsion, allow the skin to dry before applying the dressing.
- Sterile individual packaging, for single use: reuse of a disposable dressing can lead to risks of infection.
- Do not re-sterilise the dressing.
- Check that the sterility protector is intact before use. Do not use if the packaging is damaged.
FORMATS
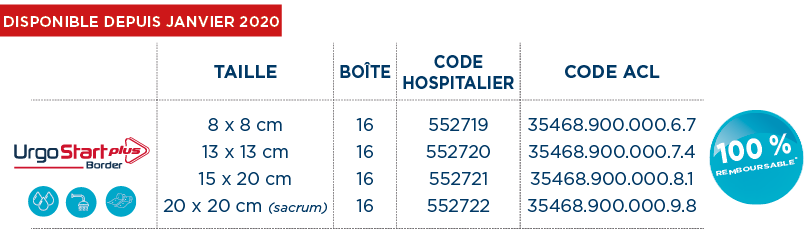
*Dispositifs Médicaux de classe IIb (GMed 0459). Traitement pour réduire le temps de cicatrisation. Intégralement remboursables LPPR (Sec. Soc. : 60% + Mutuelle : 40%) dans le traitement de l’ulcère de jambe veineux ou mixte à prédominance veineuse, en phase de bourgeonnement (traitement séquentiel), et dans l’ulcère du pied chez le patient diabétique d’origine neuro-ischémique (ischémie non critique), non infecté (critères d’infection IDSA/IWGDF), en phase de bourgeonnement (traitement séquentiel).
CLINICAL EVIDENCE
TLC-NOSF, AN INNOVATION WITH PROVEN AND RECOGNISED EFFICACY

In 2012, the Journal of Wound Care published the CHALLENGE(5) study, demonstrating that TLC-NOSF treatment leads to a greater reduction in wound surface area compared to a neutral dressing.
In December 2018, The Lancet Diabetes & Endocrinology international medical journal published the EXPLORER(2) clinical trial, demonstrating that TLC-NOSF treatment heals 60% more patients compared to a neutral dressing and that the earlier TLC-NOSF treatment is started, the more effective it is(3).
 The REALITY(4) analysis, published in 2017 in the Journal of Wound Care, presents a compilation of eight observational studies conducted in more than 10,000 patients and shows that TLC-NOSF reduces the healing time of chronic wounds by an average of 100 days and that the earlier TLC-NOSF treatment is started, the more effective it is.
The REALITY(4) analysis, published in 2017 in the Journal of Wound Care, presents a compilation of eight observational studies conducted in more than 10,000 patients and shows that TLC-NOSF reduces the healing time of chronic wounds by an average of 100 days and that the earlier TLC-NOSF treatment is started, the more effective it is.
In 2020, the Journal of Wound Care published the last URGOSTART PLUS OBSERVATIONAL STUDY conducted in 1,140 patients, demonstrating that UrgoStart Plus is effective irrespective of the wound type, whatever the wound phase and however long it has been present(6).
![]() The HAS (French National Authority for Health) has granted the entire range a level III clinical added value (CAV)(7) something that is unprecedented for a dressing.
The HAS (French National Authority for Health) has granted the entire range a level III clinical added value (CAV)(7) something that is unprecedented for a dressing.

The URGO Group was awarded the 2018 Galien France medical device prize for UrgoStart®. Each year, this prestigious award recognises exceptional innovations in the field of health.

In January 2019, the United Kingdom’s NICE (National Institute for Health and Care Excellence) recommended TLC-NOSF treatment for the wound care of diabetic foot ulcers and venous leg ulcers(8). The NICE recommendations support the fact that the UrgoStart® range has better results in terms of reducing wound healing time, improving patients’ quality of life and enabling significant savings for health authorities compared to neutral dressings.

2019: IWGDF (International Working Group on the Diabetic Foot) guidelines This is the very first time that a dressing has been recommended by the IWGDF.(9)